The process of fluorescence in diamonds begins with the formation of the diamond itself. Diamonds are created deep within the earth, under intense heat and pressure, from the carbon atoms of organic matter. As the diamond grows and forms, trace elements such as nitrogen, hydrogen, and boron may become incorporated into its crystal structure.
The Color Depends On The Elemental Composition
These trace elements act as impurities within the diamond, and can affect its optical properties, including its fluorescence. The specific color of the fluorescence is determined by the type and concentration of the trace elements present within the diamond. For example, diamonds with high levels of nitrogen may exhibit a yellow or orange fluorescence, while those with higher levels of hydrogen may exhibit a blue fluorescence.

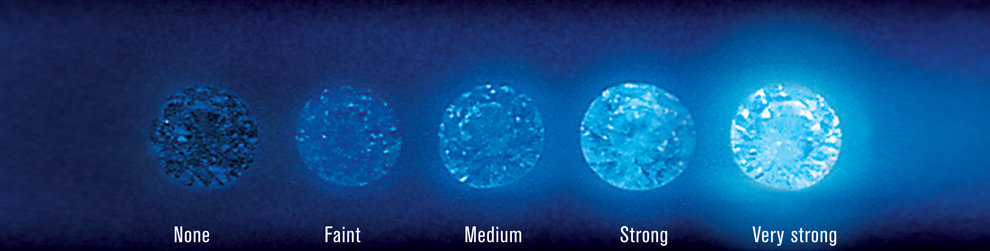
The picture above shows how diamonds look under UV light.
Only Really Visible Under Ultraviolet Light
Once the diamond has formed, it must be exposed to UV radiation in order for the fluorescence to be visible. This can happen naturally, when the diamond is exposed to sunlight or other sources of UV radiation. Alternatively, the diamond can be artificially exposed to UV radiation using a specialized light source, such as a UV lamp or black light.
When the diamond is exposed to UV radiation, the trace elements within the diamond absorb the energy and become excited. This excitation causes the electrons within the trace elements to move to a higher energy state, and as they return to their normal state, they emit light. The specific color of the light emitted depends on the type and concentration of the trace elements within the diamond.
It’s Not Black And White
In some cases, fluorescence in diamonds can affect their overall appearance and value. While some people may find the fluorescence to be attractive, others may find it to be distracting or even unattractive. Additionally, the intensity of the fluorescence can also play a role in the diamond’s value. Diamonds with a strong fluorescence may appear hazy or oily under certain lighting conditions, which can reduce their overall clarity and sparkle.
However, it is important to note that not all diamonds exhibit fluorescence, and in many cases, the fluorescence is not strong enough to affect the diamond’s appearance or value. In fact, many diamonds with fluorescence are considered to be of high quality and are highly sought after by collectors and jewelers.
Overall, the process of fluorescence in natural diamonds is a complex and fascinating phenomenon that is influenced by the trace elements present within the diamond and the amount of UV radiation it is exposed to. While fluorescence can sometimes affect the diamond’s appearance and value, it is also an important characteristic that can be used to identify and evaluate the quality of a diamond.
But There Are Still Some Visual Effects Under The Sun: Fluorescence Under Sunlight
Sunlight contains a small amount of UV light, which can cause diamonds to fluoresce. The strength of the fluorescence depends on the amount of UV light that the diamond is exposed to and the strength of the fluorescence in the diamond itself. Diamonds with strong fluorescence may appear hazy or milky in sunlight, while diamonds with faint fluorescence may not show any visible effect.
There are several factors that can affect the strength of diamond fluorescence, including the type of diamond, the size of the diamond, and the quality of the cut. Diamonds with a yellow or brown tint are more likely to fluoresce than diamonds with a colorless appearance. Larger diamonds are also more likely to fluoresce than smaller diamonds.